Research
Diffusion in metals.
Elementary mechanisms of diffusion in metals are studied on the example of PdAg nanoalloy. A uniform nanoalloy has been obtained with reversible and repeatable surface segregation (Pd or Ag) during heating at 400 deg.C in changing atmosphere (He or CO) (Kaszkur, Juszczyk, Łomot, Phys. Chem. Chem. Phys., 2015, 17, 28250).
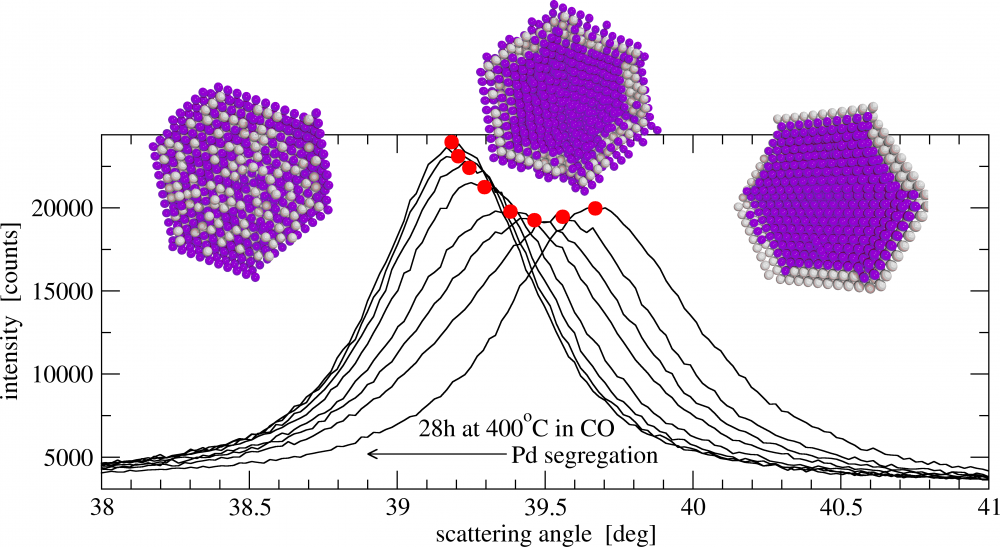
111 alloy peak evolution during Pd segregation at 400 deg.C in CO atmosphere. The models above illustrate calculated element distribution from Monte Carlo simulations.
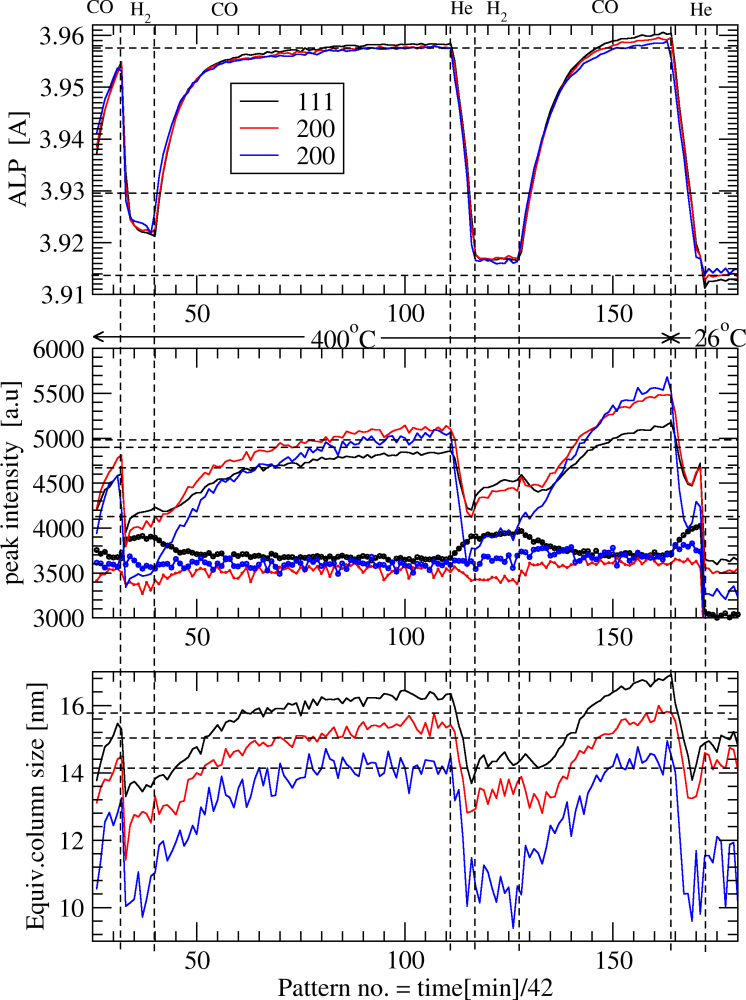
Evolution of 111, 200, 220 peak parameters on repeatable exposure to H2, CO, He.
The phenomenon of strong adsorption of CO onto Pd can be employed to generate uniform PdAg alloys. If sintering can be avoided, the nanoalloy can be subjected to repeatable processes of segregation of Pd and then Ag to the surface. The rate of both diffusion phenomena differs substantially. An atomistic understanding of them suggests a quite different mechanism for both as Pd diffusion to the surface runs in an environment depleted of vacancies. This recalls an old discussion concerning the diffusion basics before discovery of the Kirkendall effect. We observed that diffusion transport is slower than the bulk diffusion reported in the literature.
Evidently the macroscopic diffusion involves different mechanisms with diffusion along grain boundaries, dislocations and the effect of impurities. A study of segregation phenomena in nanocrystals can allow an insight into more elementary lattice transport. Switching off the dominating vacancy mechanism of diffusion allows us to study other subtle transport phenomena in alloys.