Research
Research overview 2013
V. Department of Catalysis on Metals
Contact: Zbigniew Kaszkur – professor of the Institute of Physical Chemistry PAS, zkaszkur@ichf.edu.pl
Dynamics of nanocrystal structure induced by surface chemistry
(Z.Kaszkur group)
Laboratory of X-ray Powder Diffraction and Spectrometry has a long standing experience in in situ powder diffraction as well as EXAFS (Extended X-ray Absorption Fine Structure) studies [eg. 1,2]. For in situ diffraction the employed equipment includes a range of 'in lab' constructed environmental cameras (J.Zieliński) as well as position sensitive detector INEL CPS120 (fig.1). The group undertook also successful combined XRD- Impedance Spectroscopy studies with a specially constructed in situ XRD cell [3].
Since 1997 the laboratory began research leading to development of a new powder diffraction in situ technique allowing surface science studies of nanocrystals in a chemical reaction conditions. The recent proof-of-concept work [4] shows in situ monitoring of a surface reconstruction of Pt nanocrystal surface (fig.2). On the development path the laboratory has showed deviations from Bragg law due to nanocrystallinity- pure size effect and a surface relaxation effect. The first diffraction experimental observation of surface relaxation of Pd nanocrystals has been published in 2000 [5] parallely with development of molecular simulation tools to interpret the observed diffraction phenomena [6]. The developed tools are available as program CLUSTER (Kaszkur, Mierzwa) - simulation package including graphical interface (fig.3).
The developed tools were applied to interpret phenomena of surface segregation in nanocrystalline bimetallic alloys [7,8] including reversal of the concentration profile in changing gaseous environment. Also interpretation of changing diffraction profiles on exposition of nanocrystalline Pd to hydrogen, proving existence of non-forming hydride phase icosahedral Pd clusters, was possible with the developed software.
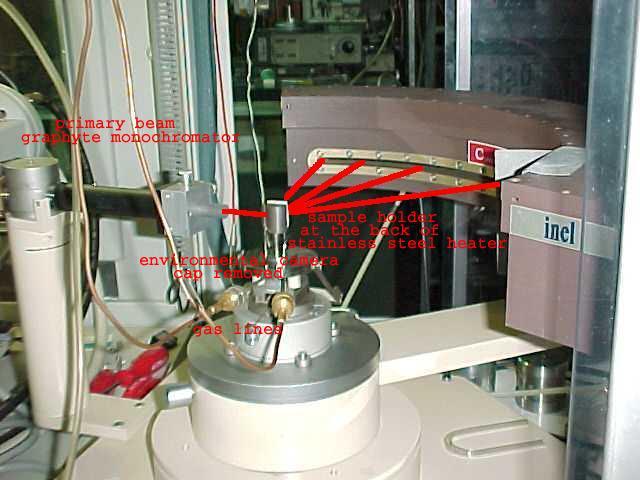
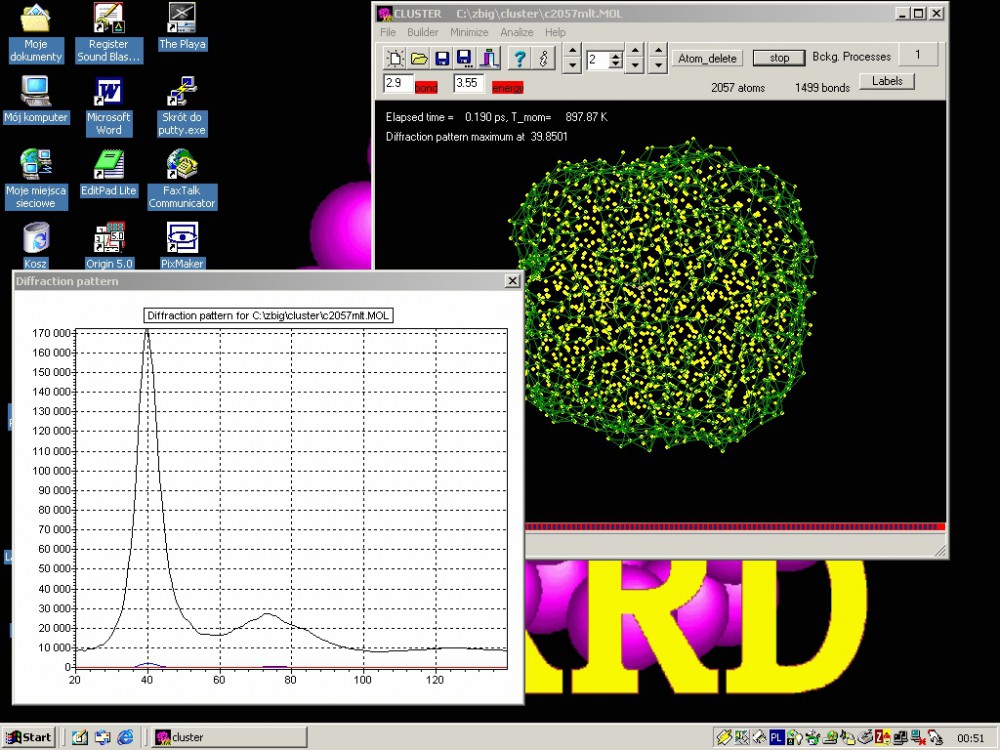
Fig.1. Position sensitive detector INEL CPS120 with environmental camera (cap removed).
Fig.3. Layout of the program CLUSTER
A large scale atomistic modeling enabling simulation of thermodynamics of metal clusters build of hundreds thousand atoms serves also as a testing field for many phenomenological descriptions of the diffraction phenomena. Stacking faults, dislocations, strain and various cluster shapes can be imposed on a model and provide a model powder diffraction pattern that can be further analyzed with current phenomenological methods evaluating their accuracy. This enabled evaluation of applicability of various powder diffraction analytical tools to nanocrystals as small as 2-10nm [9], as well as development of a classic Warren-Averbach peak profile analysis method [10].
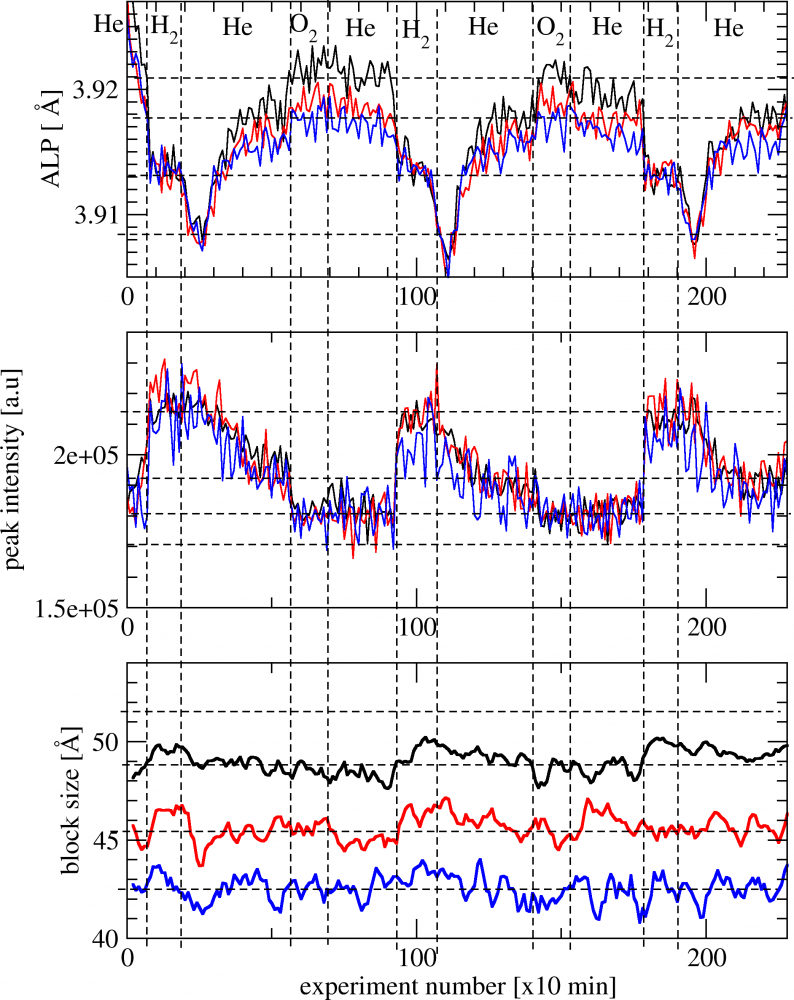
Fig.2. Cycles of surface reconstruction of Pt nanocrystals in He after reduction in H2. Apparent lattice parameter (ALP- upper curve) from position of the diffraction peak: 111 -black, 200 -red, 220 -blue line. After exposition to helium the pattern spontaneously evolves over few hours with detectable change in peak position and intensity [3]
Another part of the group activity was devoted to investigate interaction of nanocrystalline nickel with hydrogen using temperature-programmed desorption (TPD) technique (J.Zieliński, L.Znak [11,12]). The study was performed in a flow system, starting the TPD measurements at 78 K, which resulted in recording of three forms of chemisorbed hydrogen: α, adsorbed on Ni surface, β, adsorbed in the “second layer”, and γ, located in the subsurface region of nickel (fig.4). It was the first in the literature observation of the low temperature γ phase for adsorption of molecular hydrogen.
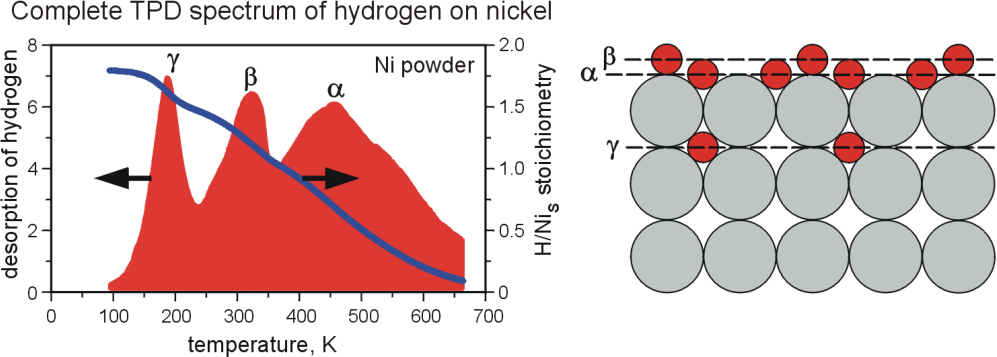
Fig.4. The temperature-programmed desorption (TPD) results for Ni/SiO2 catalysts.
- Z.Kaszkur, J.Stachurski, J.Pielaszek, J.Phys.Chem.Solids, 47 ,795(1986).
- B.Mierzwa, Z.Kaszkur, B.Moraweck, J.Pielaszek, J.Alloy.Comp., 286, 93-97(1999).
- Kopeć M., Lisovytskiy D., Marzantowicz M., Dygas J.R.,Krok F., Kaszkur Z., Pielaszek J., Journal of Power Sources, 159, 412-419 (2006).
- P.Rzeszotarski, Z.Kaszkur, Phys.Chem. Chem.Phys., 11, 5416-5421 (2009).
- Z.Kaszkur, J.Appl.Crystallogr., 33, 1262-1270 (2000).
- Z.Kaszkur, J.Appl.Crystallogr., 33, 87-94 (2000).
- Z.Kaszkur, Phys.Chem.Chem.Phys., 6, 193-199 (2004).
- Z.Kaszkur, Philos.Mag., 77, 781-800(1998).
- Z.Kaszkur, Zeit.Kristallogr., 23, 147-154 (2006).
- Z.Kaszkur, B.Mierzwa, J.Pielaszek, .J.Appl.Crystallogr., 38, 266–273 (2005).
- L. Znak, J. Zieliński, Langmuir, 22, 8758-8763 (2006).
- L. Znak, J. Zieliński, Appl. Catal. A: Gen. 324 (2008) 268.