Funding
National Science Centre Grant SONATA 9 no. UMO-2015/17/D/ST4/00112
Iron-sulfur clusters as natural switches: consequences of the unique iron atom coordination at a molecular level.
Project leader: dr Adam Kubas
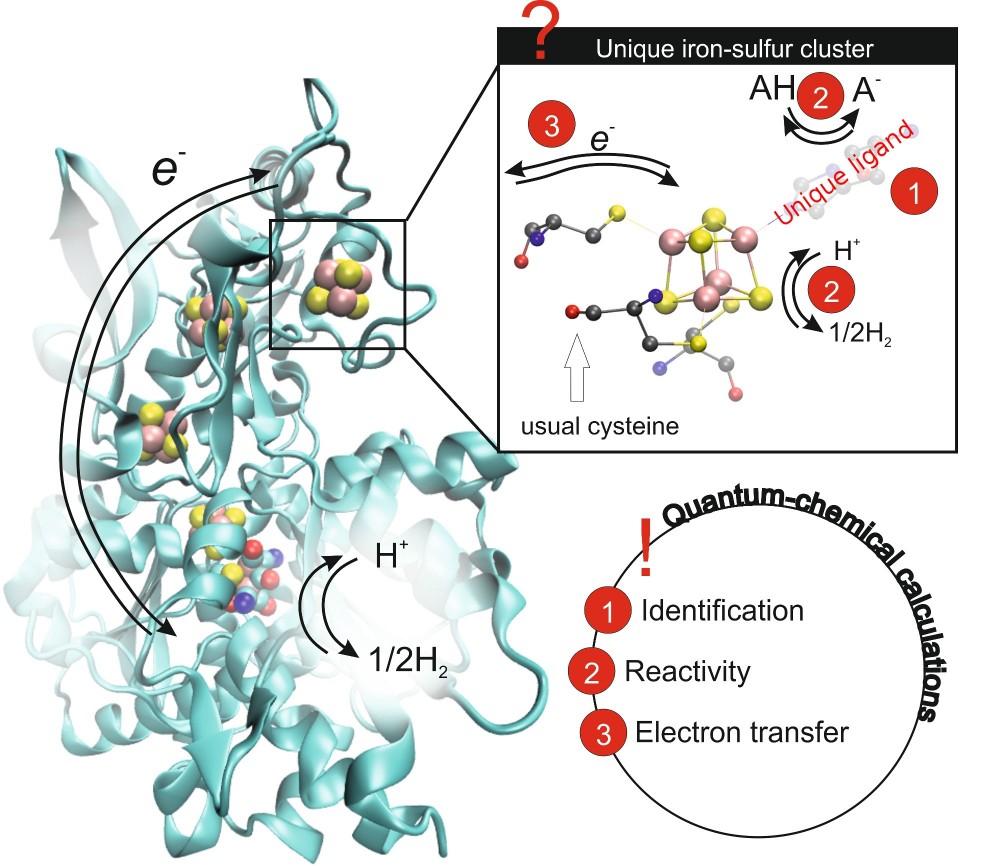
The production of H2 based on the enzymatic catalysis, in contrary to industrial-scale production methods, avoids unwanted by-products like CO2 and high energy consumption. While it is already possible to incorporate entirely synthetic active centre into a protein matrix, the way how nature switches between oxidation and reduction reactions, i.e. changes the electron flow direction, remains unclear. The project aims to improve our understanding of the electron flow regulation in enzymes relevant to hydrogen production (hydrogenases). The impact of unique ligands and their protonation state on the [Fe4S4] clusters that form electron transport chains can be used to design hybrid natural-synthetic systems with fine-tuned redox characteristics. The possibility of usage of these clusters in a direct H2 production is an attractive perspective. The prerequisite for such applications is a detailed knowledge of the influence of all molecular factors on the electron transport properties and H-H bond formation reaction. The project will explore theoretically this direction of [Fe4S4] clusters utilisation and provide insides into both processes. It will go one step further to check if naturally occurring iron ligands like histidine or aspartate can ease the reaction. Moreover, X-ray absorption and emission spectra obtained in this study can be directly compared with experimental data in order to unambiguously determine entire coordination sphere of any [Fe4S4] cluster in a protein. In this way, the long-standing question of the fourth ligand of hydrogen assembly protein HydF can be answered.



