Research
Bioinspired intelligent molecularly imprinted polymers (MIPs) for selective chemosensing
Receptors found in living organisms specifically recognize target biomolecules. If applied to biosensing, they offer the much desired high selectivity. However, their instability under measurement conditions and low durability prompted researchers toward devising different artificial receptor alternatives. For instance, an antibody is successfully mimicked by a corresponding molecularly imprinted polymer (MIP), sometimes called a “plastic antibody”.
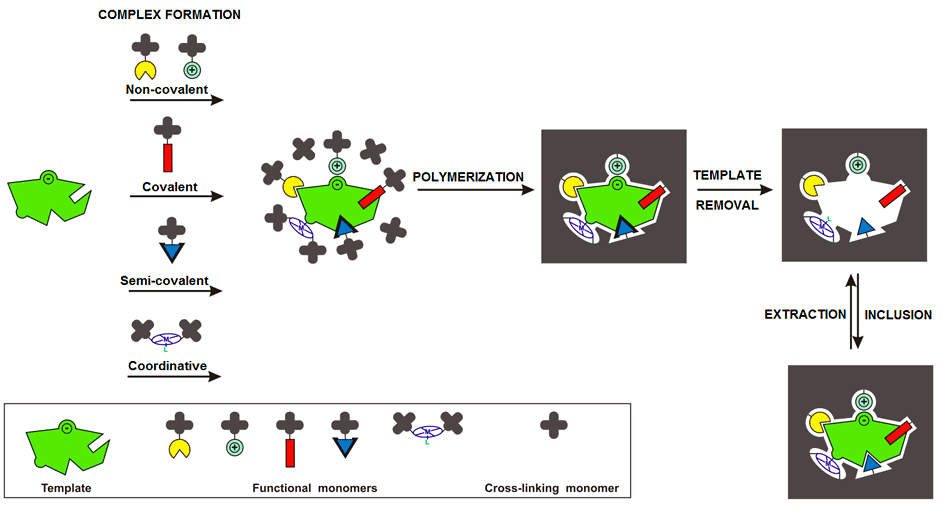
Scheme 1. A sketch of consecutive steps of preparation of a molecularly imprinted polymer (P. S. Sharma, M. Dabrowski, F. D’Souza, W. Kutner, Surface development of molecularly imprinted polymer films to enhance sensing signals, TrAC, Trends Anal. Chem. 51 (2013) 146–157)
Nowadays, MIPs are widely used, among others, as recognition units in chemical sensors. Molecular imprinting involves impressing molecular cavities in a polymer matrix with template molecules. The cavity shape, size, and orientation of recognition sites generated in these cavities match the shape, size, and orientation of binding sites of the template molecule (Scheme 1). The analyte itself or its close analogue is selected as this template. The imprinting procedure involves, first, pre-organization of the template with functional monomers to form a complex in solution followed by polymerization of this complex in the presence of a cross-linking monomer. Subsequent removal of the template from the resulting rigid polymer matrix leaves behind imprinted cavities complementary to the template molecules. These cavities can be imprinted for both small molecules and macromolecules, such as proteins and peptides.
Our research is focussed on the development of novel recognition materials based on MIPs for devising chemosensors selectively determining biomarkers, toxins, and biomolecules. We achieve the desired selectivity in our devised chemosenors because of the presence of multiple modes of reversible binding of molecules of the target analytes in tailored designed molecular cavities.
Todate, we have successfully integrated different MIPs on several transducer surfaces, such as those electrochemical, electrical, optical, and piezomicrogavimetrical. Because of ease of preparation, we exclusively use electropolymerization for preparation of both conducting and non-conducting MIP films. Towards that, electropolymerizable bis(2,2’-bithienyl)methane electroactive functional monomers derivatized with different built-in molecular recognition sites are designed and synthesized by our partner, Prof. F.D’Souza (Chemistry Department, University of north Texas, Denton, USA) (Scheme 2).
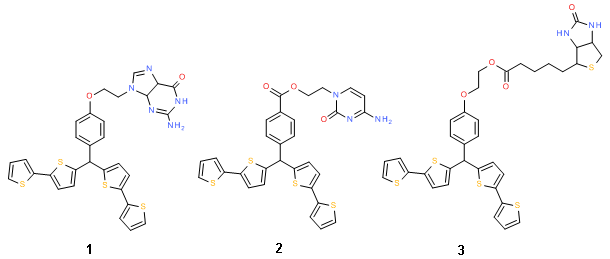
Scheme 2. Structural formulas of examples of the designed functional monomers, p-bis(2,2'-bithien-5-yl)methylphenol 2-(guanin-9-yl)ethyl ether 1, 2-(cytosin-1-yl)ethyl p-bis(2,2'-bithien-5-yl)methylbenzolate 2, 2-[p-bis(2,2'-bithien-5-yl)methylphenoxy]ethyl biotin ester 3.



