Research
Our offer of scientific instrumentation: hanging mercury drop electrode, HMDE WK 2
General description and application
The hanging mercury drop electrode HMDE WK 2 (named after Prof. Wiktor Kemula) is a spherical stationary mercury mini electrode with surface area of the order of a square millimeter.1
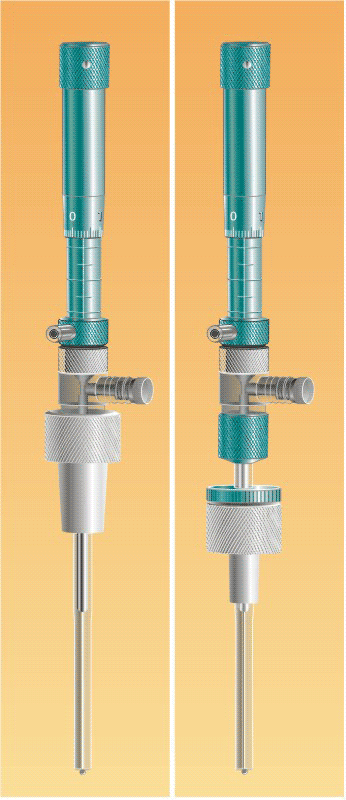
The hanging mercury drop electrode HMDE WK 2 (named after Prof. Wiktor Kemula)
A mercury drop is suspended at the end of a glass capillary and its size is conveniently adjusted with a micrometer screw. The electrode HMDE WK 2 can be used as a working electrode in various electroanalytical techniques for studying mechanisms and kinetics of electrochemical reactions as well as for determination of analytes in an electrolyte solution.
Construction
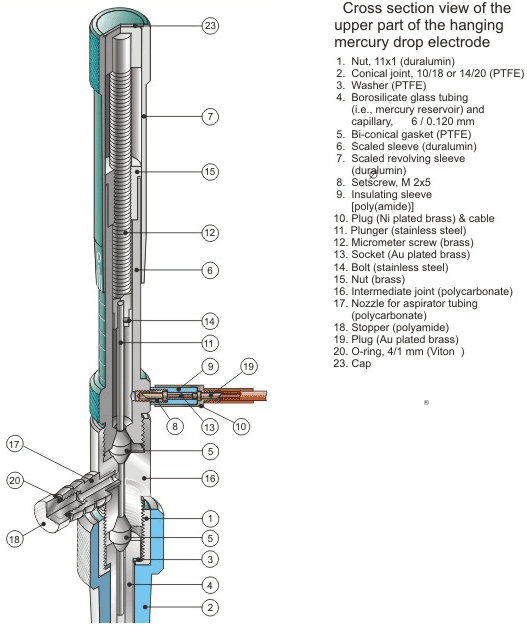
Figure 1.
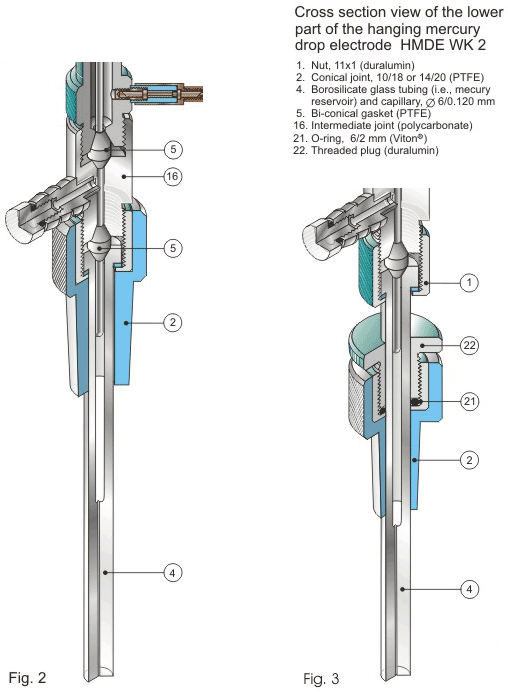
Figures 2 and 3.
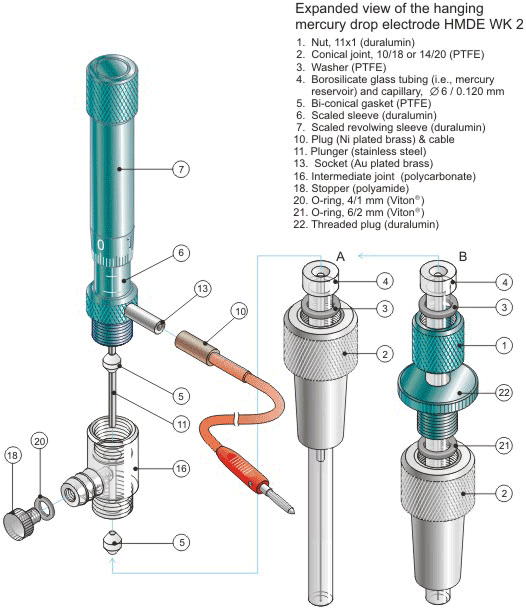
Figure 4.
The electrode HMDE WK 2 features a very reliable mercury sealing system provided by two bi-conical gaskets (Parts 5 in Figs. 1 and 2). The combination of this system with a micrometer screw (Part 12 in Fig. 1), used for mercury proportioning, results in exceptional reproducibility of a mercury drop surface area. Construction and design of the electrode HMDE WK 2 are greatly improved over the former electrode HMDE WK 1.2 That is, the electrode weight and dimensions are smaller while the number of mercury drops (available from a single electrode filling with mercury) is larger. This is because the plunger (Part 11 in Fig. 1) is longer and its diameter is larger. Therefore, a working mercury volume is about six times larger; the mercury reservoir being only slightly longer.
Importantly, the HMDE WK 2 can be mounted in two different configurations of terminal assemblies (Figs. 2-4). One of them involves a 10/18 or 14/20 PTFE conical sleeve (Part 2 in Figs. 1, 2, and 4A) attached directly to the translucent intermediate joint (Part 16 in Figs. 1, 2 and 4) that allows one to house the electrode HMDE WK 2 in a typical commercial electrochemical cell. The other configuration involves a duralumin nut (Part 1 in Figs. 3 and 4B). A thermometer type PTFE adjustable adapter for a standard tapered glass joint 10/18 or 14/20 (Parts 2, 21 and 22 in Figs. 3 and 4B) is then used for housing the electrode in a cell. The main body of the electrode HMDE WK 2 is made of electrochemically colored duralumin. Both the mercury reservoir and the precision-bore capillary (Part 4 in Figs. 1-4) are made of borosilicate glass.
Features
| Total length | 240 mm |
| Outer diameter | 14 mm |
| Weight | 52 g |
| Plunger diameter | 1.6 mm |
| Working length of plunger | 45 mm |
| Pitch of thread | 0.35 mm |
| Glass capillary inner diameter | 0.127 mm or 0.178 mm |
| Glass tubing and capillary length | 100 mm (or customized) |
| Volume of working mercury | 96.5 mm3 |
| Reproducibility of the electrode surface areaa | 0.60 % |
aDetermined as relative standard deviation of a peak current of the linear potential scan voltammetry curves for the Cd2+/Cd(Hg)0 electroreduction in 1 mM Cd(NO3)2, 0.5 M KCl for an electrode area of ca. 2 mm2 (Fig. 7).
Assembling the electrode
The electrode delivered to a customer is assembled and filled with mercury. But for changing the capillary (4), the electrode must be re-assembled. For that purpose, first, the assembling configuration, depicted in Figs. 4A or 4B, has to be selected. Then, a new capillary as well as a new lower bi-conical gasket (5) should be mounted. For that purpose, initially, the plunger (11) should be raised to its upper position with the scaled revolving sleeve (7) and both threads of the intermediate joint (16) left loose (Fig. 5a).
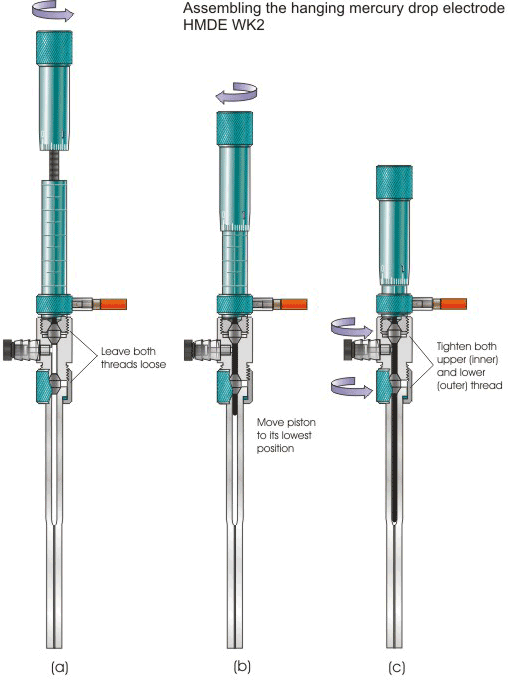
Figure 5.
Note 1. When changing the capillary, always use a new lower bi-conical gasket (5).
Next, move the plunger (11) to its lowest position (Fig. 5b). Subsequently, tighten firmly (Fig. 5c) the upper (inner), then, lower (outer) thread of the intermediate joint (16).
Initial filling the electrode with mercury
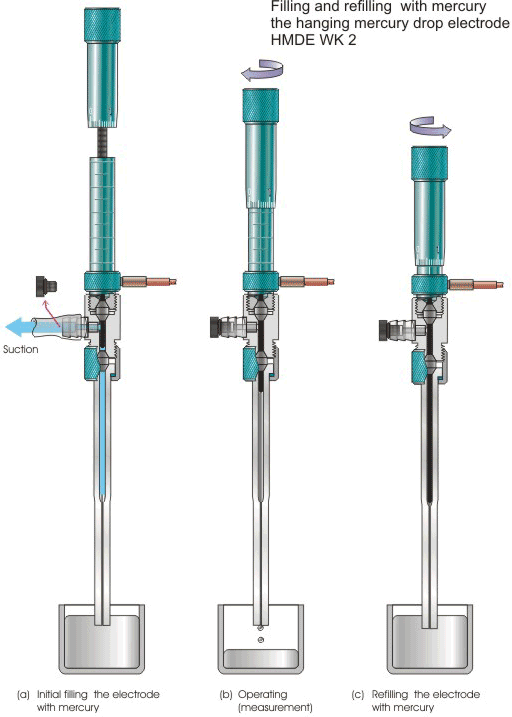
Figure 6.
The use of the intermediate joint (16) allows one for initial filling the electrode HMDE WK 2 with mercury by using an "overflow" procedure (Fig. 6). In this procedure, the stopper (18) should be removed and suction, afforded with, e.g., water aspirator, applied to the nozzle (18) while the capillary tip immersed in a mercury pool and the electrode held vertically (Fig. 6a). The electrode can be filled if the plunger is raised above the lower gasket (5), as viewed through the intermediate joint, by turning counter clockwise the scaled sleeve (6). When the mercury level is raised by suction above the lower gasket (5), move the plunger below the lower gasket (5) and, then, shut off the suction (Fig. 6b).
Note 2. In order to prevent air entering the mercury reservoir and, hence, malfunctioning the electrode, first, move the plunger (11) below the lower gasket (5), i.e., to reach a level of ca. 3.5 main mark of the scaled sleeve (6) and, then, shut off the suction.
Finally, replace the stopper (18).
Refilling the electrode with mercury
For refilling the electrode with mercury (Fig. 6c), immerse its capillary tip in a mercury pool and turn counter clockwise the scaled revolving sleeve (7) while holding the electrode vertically.
Note 3. Suction is not needed for refilling the electrode with mercury. Make sure that the tip of the plunger (11) remains below the lower gasket (5), i.e., the scaled revolving sleeve (7) is raised to reach not higher than ca. 3.5 main mark of the scaled sleeve (6).
Testing the electrode
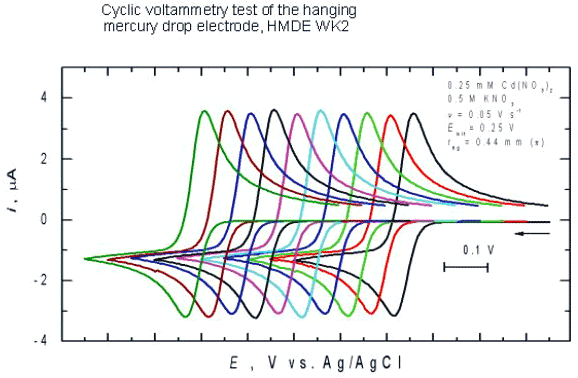
Figure 7.
It is recommended to test the electrode under, e.g., cyclic voltammetry conditions, before using it for research purposes, as illustrated in Figure 7. In this figure, 10 consecutive cyclic voltammograms are presented. Each voltammogram was recorded at a fresh mercury drop formed by turning clockwise the scaled revolving sleeve (7) by 180°.
Tap gently at the cap (23) with your forefinger in order to detach a mercury drop. After the drop falls, a mercury column front inside the capillary (4) may raise above the end of the capillary by a few mm. Therefore, bring the mercury column front to the end of the capillary with the micrometer screw before extruding the next drop for the best drop size reproducibility.
Caution. Mercury is a poison. If you swallow it accidentally, then contact your physician immediately.
References
-
Głód, B. K., Kemula, W., Chem. Anal. (Warsaw), 1985, 30, 665.
-
Kemula, W., Zawadowska, J., Fresenius Z. Anal. Chem., 1980, 300, 39.
For further details go to:
Institute of Physical Chemistry
Polish Academy of Sciences
Kasprzaka 44/52
01-224 Warsaw
Poland
Tel.: +(48 22) 343 3217 or +(48 22) 343 2094
Fax: +(48 22) 343 3333 or +(48 22) 632 5276
wkutner [at] ichf.edu.pl, mdabrowski [at] ichf.edu.pl



