Research
Mechanism of disruption of model bacterial cell membranes by antimicrobial peptides
Our efforts are directed towards understanding the mechanism of disruption of model bacterial cell membranes by antimicrobial peptides (AMPs) and the role of cholesterol in protecting mammalian-like membrane from the AMPs attack.
Planar lipid systems, such as supported bilayers, are useful models for studying interactions of membranes with antimicrobial AMPs. Planar systems generally resemble lateral organization of phospholipids in cellular membranes. In fact, they can be considered as superior mimics of actual cell surfaces on a molecular scale due to their non-existent surface curvatures. We investigate interactions of cholesterol and AMPs with lipid bilayers by using Electrochemical Scanning Tunneling Microscopy (EC-STM) and Atomic Force Microscope (AFM). Conformational and orientational changes of phospholipids and AMPs in the supported bilayer are studied by Polarization Modulation Infrared Reflection Absorption Spectroscopy (PM-IRRAS). Planar lipid bilayers (SLBs) are immobilized onto a planar substrate by the Langmuir-Blodgett or Langmuir-Schaffer deposition or by fusion and spreading of small, unilamellar vesicles (Figure 1).
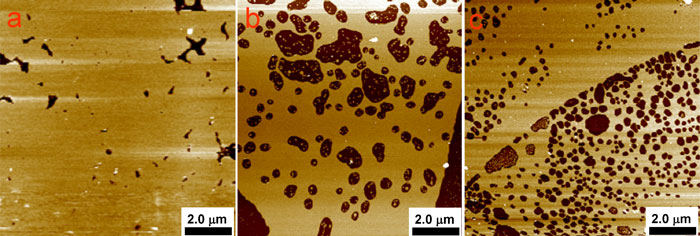
Figure 1. Atomic Force Microscopy images of the DMPC/Chol (7:3 mol ratio) bilayers deposited by (a) SUVs fusion, (B) LB-LB, i.e. both first and second monolayer was transferred by using Langmuira-Blodgett method, (c) LB-LS, i.e., first and second monolayer was transferred by Langmuir-Blodgett and Langmuir-Scheafer method, respectively.
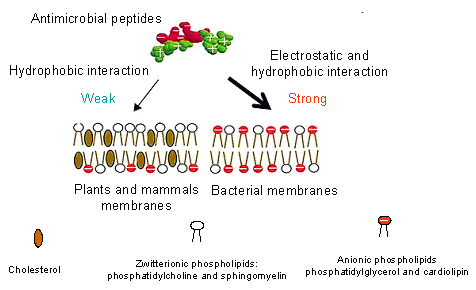
Scheme 1. Membrane composition presumably accounts for the difference in AMP susceptibility.
AMPs show different affinities towards the bacterial and mammalian cell membranes. It is believed that they are able to interact selectively with bacterial membranes, which contrary to plants and mammals membranes, are negatively charged due to the presence of anionic phospholipids such as phosphatidylglycerol (PG) or cardiolipin in the inner layer (Scheme 1). Additionally, the cholesterol, which is only present in mammalian cells as a membrane stabilizing agents, protects the cells from the antimicrobial peptides attack. The last factor that affects the selectivity of AMPs is the transmembrane potential, which facilitates membrane permeabilization by enhancing affectivity of the positively charged peptides insertion into membranes. This transmembrane potential is more negative for bacterial cells than that for mammalian cells, promoting attack by the cationic AMPs. AMPs, most likely, interact with bacterial membranes mainly via electrostatic interaction, which is considered as the major driving force for cellular association. This interaction induces insertion of the peptide into a position parallel to the membrane at the interface of the hydrophilic head groups and hydrophobic fatty acyl chains of the phospholipids membrane. This parallel insertion leads to a collapse of the transmembrane electrochemical gradient and, in consequence, bacterial cell death. Several models have been proposed to describe the activity of AMPs, like the carpet model, the barrel-stave model, the toroidal model, the aggregate channel model.
Alamethicin, Alm, is a 20-residue peptide isolated from the Trichoderma viride fungus.1 Structural analysis of Alm by X-ray crystallography2 and NMR spectroscopy3 indicates that the molecule is predominantly a-helical with a bend at an internal proline residue. The helix structure is highly amphipathic because most of the polar residues are at the C terminus or are positioned along a narrow strip parallel to the helix axis. Alm peptides have been thoroughly studied mostly because they are considered as models of channels formed in biological membranes. Supposedly, Alm spontaneously inserts into a lipid membrane producing a voltage-gated channel composed of at least three4 or four subunits.5
Our EC-STM studies clearly demonstrate that the Alm molecules form flower-like channels in the phospholipid membrane (Figure 2).6
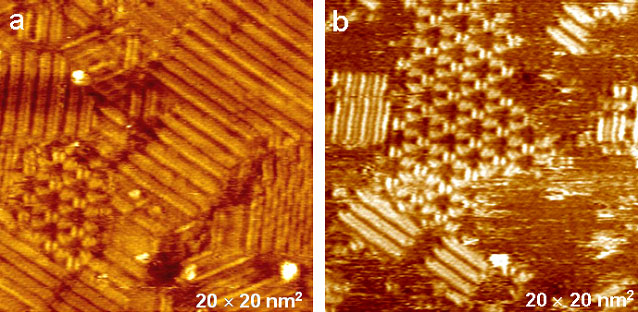
Figure 2. Electrochemical STM (EC-STM) images of a monolayer of Alm and DMPC/egPG (1:15 molar ratio) deposited on an Au(111) surface. Images were acquired using tunneling currents of (a) 1.16 and (b) 0.78 nA
References
-
1. Dittmer, J.; Thogersen, L.; Underhaug, J.; Bertelsen, K.; Vosegaard, T.; Pedersen, J. M.; Schiott, B.; Tajkhorshid, E.; Skrydstrup, T.; Nielsen, N. C., J. Phys. Chem. B 2009, 113, (19), 6928-6937.
-
2. Fox, R. O.; Richards, F. M., Nature 1982, 300, 325.
-
3. Yee, A. A.; Babiuk, R.; O’Neil, J. D. J., Biopolymers 1995, 36, 781.
-
4. Hanke, W.; Boheim, G., Biochim. Biophys. Acta 1980, 596, 456.
-
5. Sansom, M. S. P., Prog. Biophys. Mol. Biol. 1991, 55, 139.
-
6. Pieta, P.; Mirza, J.; Lipkowski, J., Proc. Natl. Acad. Sci. U. S. A. 2012, 109, (52), 21223-21227.



