Research
Organized ultrathin films of organic and organic-inorganic hybrid materials for chemical sensors
The Langmuir and Langmuir-Blodgett techniques allow preparing molecular monolayers and their transfer onto solid substrates. These simple but powerful tools make preparation of ordered mono- and multi-molecular layers easy. First, an amphiphilic compound is dissolved in a volatile solvent, and then a certain volume of this solution is dispensed dropwise on the cleaned surface of a polar liquid subphase filling a Langmuir trough (Scheme 1).1
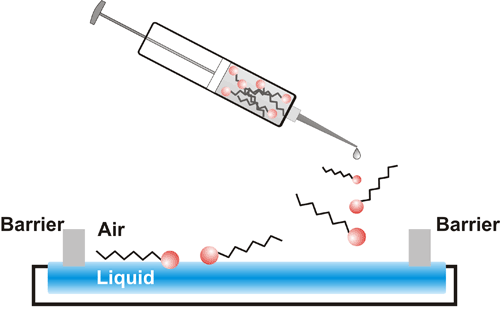
Scheme 1. Drop dispensing of amphiphilic compounds dissolved in a volatile solvent onto a polar liquid subphase filling a Langmuir trough.
After spreading the compound, the barriers of the Langmuir trough start approaching each other, thus decreasing the area of the liquid subphase available for the spread compound. A molecular Langmuir monolayer film is forming in the liquid-air interface (Figure 1). When the surface pressure becomes too high, this film collapses, that is, molecules are squeezed out forming a bi- or tri-layer film. Surface pressure monitoring allows for control over the phase nature and/or orientation of molecules in the film. Changes in the monolayer film caused by its reorganization can be monitored by measuring surface pressure, surface potential, or by Brewster angle microscopy.
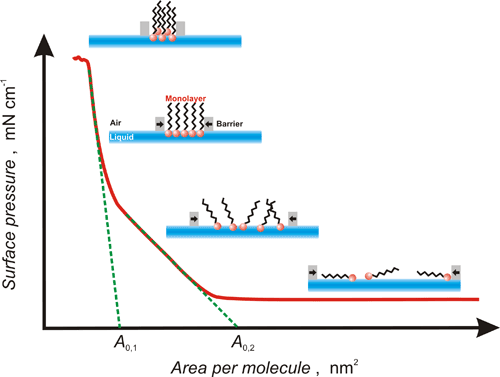
Figure 1. With the growth of the surface pressure molecules reorganize because the area of the liquid subphase available in the Langmuir trough decreases.
Monomolecular films can be transferred onto different solid substrates by several techniques; the most common is the Langmuir-Blodgett technique. It relies on adhesion of the a film to the immersed (or withdrawn) solid substrate into (or reversed) the liquid subphase (Scheme 2). Transfer of molecular films onto solid substrates allow for their further examination or application to designed use.
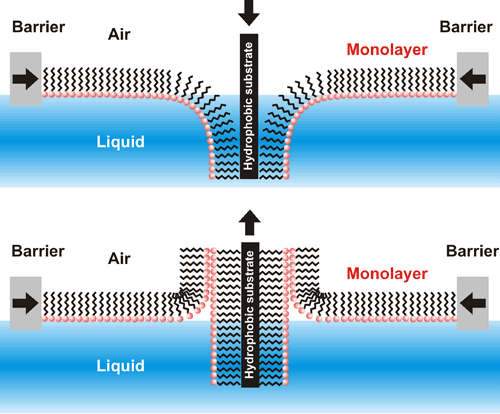
Scheme 2. The ordered molecular film can be transferred from the liquid subphase surface onto solid substrates using Langmuir-Blodgett technique to result in mono- or multi- molecular layer.
The Langmuir and Langmuir-Blodgett techniques are convenient tools for study interactions in model bio-mimicking systems. This way, bio-inspired molecular interactions may be evaluated for use in chemical sensors2,3 or liquid crystals.4 An example of these interactions is illustrated with determination of uracil nucleobase-pairing heteroatoms of biological importance (Figure 2).3
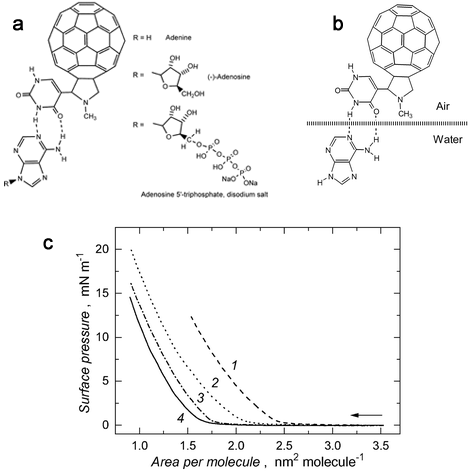
Figure 2. Molecular recognition using Langmuir films: (a) complex of uracil-appended fullerene with adenine, (-)-adenosine and ATP, (b) complex of uracil-appended fullerene and adenine, (c) Langmuir trough compression isotherms of surface pressure vs. area per molecule for a 0.2 mL sample of 0.114 mmol dm−3 C60-uracil in chloroform spread onto subphase of (1) 1.81 mmol dm−3 ATP, (2) 1.81 mmol dm−3 adenosine, (3) 1.81 mmol dm−3 adenine, (4) water. (Reproduced from R. Marczak, V. T. Hoang, K. Noworyta, M. E. Zandler, W. Kutner and F. D'Souza, J. Mater. Chem., 2002,12, 2123-2129 with permission from The Royal Society of Chemistry.)
In studying molecular recognition in two-dimentional system, selectivity of the recognition film should be carefully evaluated, because the film allows for a lot of freedom for the detected analyte. Selectivity of these films may be improved by making multilayer films or films of more complicated structure.
References
-
Ulman, A. In An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-Assembly; Academic Press: New York, 1991.
-
Obraztsov, I.; Noworyta, K.; Kutner, W.; Gadde, S.; D’Souza, F. Phys. Status Solidi Basic Res. 2007, 244, 3861–3867.
-
Marczak, R.; Hoang, V. T.; Noworyta, K.; Zandler, M. E.; Kutner, W.; D’Souza, F. J. Mater. Chem. 2002, 12, 2123–2129.
-
Milczarczyk-Piwowarczyk, P.; Zywocinski, A.; Noworyta, K.; Holyst, R. Langmuir 2008, 24, 12354–12363.



