Research
Carbon nanomaterials and polymers for application in supercapacitors
We devise and fabricate composites of multi-polymer thin films, reinforced with carbon-based nanostructure scaffolds, suitable as electrode materials for supercapacitors. We investigate in situ electrochemical, visco-elastic, and nano-mechanical properties of these composites.
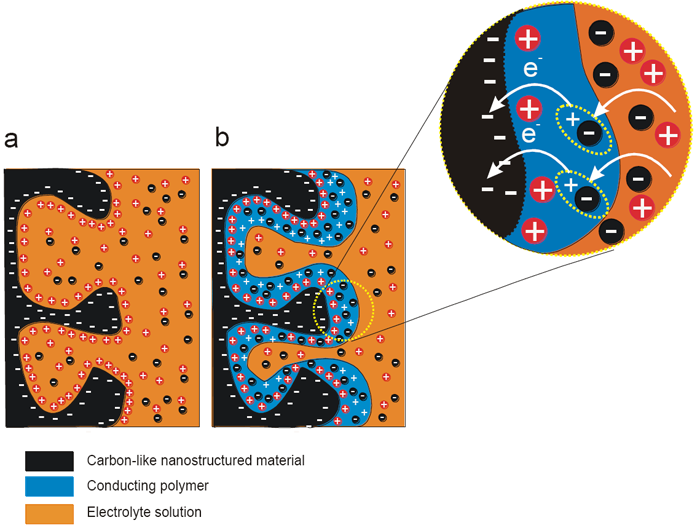
Scheme 1. Comparison of charging of an electrode of (a) a double-layer capacitor, using e.g., carbon, and (b) a pseudo-capacitor, using e.g., a conducting polymer. (Adapted from Ref.1)
In general, electrochemical capacitors store energy by two different mechanisms. Scheme 1 illustrates an energy storage mechanism, governing the charge storage (a) solely via electrostatic attraction between ions and the charged surface of an electrode substrate and (b) via electrosorption and redox reactions occurring in the electroactive material, e.g., a conducting polymer (CP) and metal oxides.1 Novel energy storage materials are fabricated by combining CPs electrochemically active in different potential ranges. That way we provide materials of highly desirable conductivity extended in a wide potential range. Both p-conducting and redox-conducting polymers are very promising for that purpose.
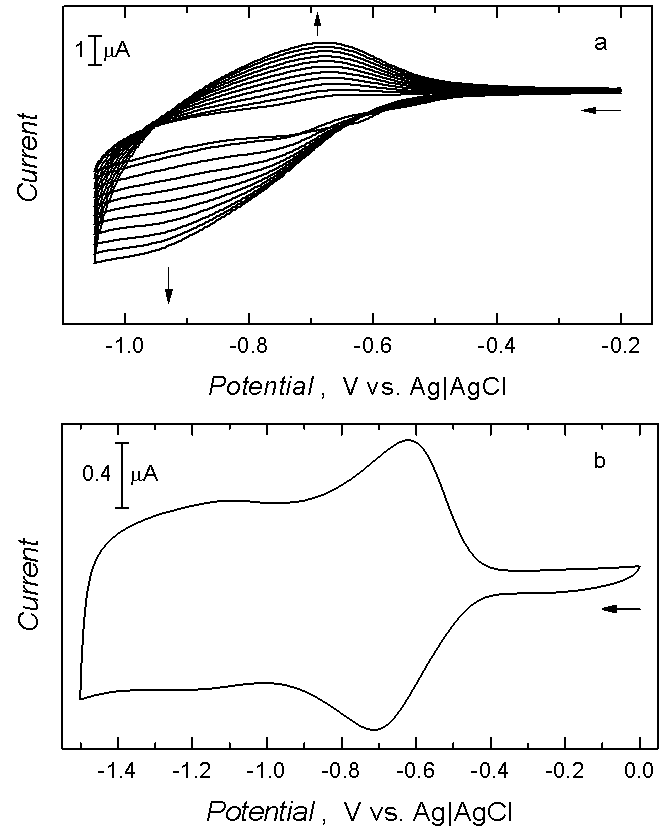
Figure 1. (a) Multi-scan cyclic voltammograms for electrodeposition of films of C60-Pd polymer in solution of 0.34 mM C60, and 2.5 mM palladium(II) acetate in toluene : acetonitrile (4 : 1, v : v) on the 0.1-mm diameter Pt disk electrode. Potential sweep rate was 100 mV/s, (b) Cyclic voltammogram for film of C60-Pd polymer in 0.1 M (TBA)ClO4, in o-DCB. Potential sweep rate was 100 mV/s. (Adapted from Ref.2)
A C60-Pd film can be formed in the course of potentiodynamic electroreduction of C60 and palladium acetate [Pd(ac)2] in the tetra(n-butyl)ammonium perchlorate (TBAP) mixed solvent solution of acetonitrile and toluene (1 : 4, v / v) by repetitive potential cycling between ‑0.20 and ‑1.05 V (Figure 1a).2 The cyclic voltammetry behavior of the resulting film in the blank acetonitrile solution of tetra(ethyl)ammonium perchlorate (TEAP) is presented in Figure 1b. The shape of the voltammogram is pseudo-rectangular in the potential range of ‑0.60 to ‑2.00 V vs. Ag|AgCl. The current measured includes capacitive and faradaic components, both linearly depending on the potential scan rate. The C60-Pd film was the most stable with respect to potential cycling for potentials below ‑1.20 V in the TEAP solution of acetonitrile.
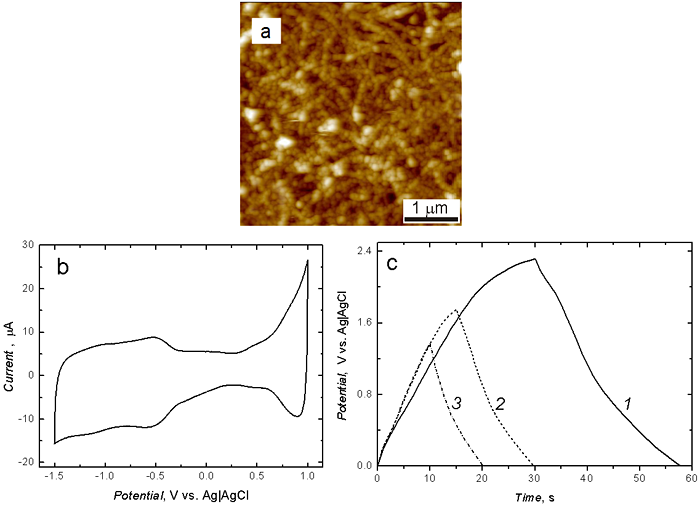
Figure 2. (a) Atomic force microscopy image, (5 x 5 mm2 area) of the film of (C60‑Pd)‑PBT, (b) Cyclic voltammogram for the film of the pyr-SWCNTs|(C60-Pd)-PBT in 0.1 M (TBA)ClO4, in acetonitrile, and (c) The charge-discharge curves for the pyr‑SWCNTs|(C60Pd)-PBT film-coated Au electrode in 0.1 M (TBA)ClO4, in acetonitrile, at constant current of 90 mA for the voltage limit of (1) 1.40, (2) 1.80, and (3) 2.30 V. The film was deposited by electropolymerization in 0.27 mM C60, 3.56 mM Pd(ac)2, 1 mM bisthiophene, and 0.1 M (TBA)ClO4, in toluene : acetonitrile (4 : 1, v : v) onto the Au-quartz|pyr-SWCNT film-coated electrode. (Adapted from Ref.2)
A co-polymer comprising both the redox active C60-based polymer and the p-conducting bisthiophene polymer can be simultaneously deposited on an electrode. First, HiPCO single-wall carbon nanotubes non-covalently surface modified with 1-pyrenebutyric acid (pyr-SWCNTs) are electrophoretically deposited on the electrode surface. Then, the C60 and bisthiophene co-polymer is deposited by potentiodynamic electroreduction of C60 in the presence of Pd(ac)2, and electro-oxidation of bisthiophene in the tetra(n-butyl)ammonium perchlorate (TBAP) mixed solvent solution of acetonitrile and toluene (1 : 4, v / v) by repetitive potential cycling between ‑1.50 and 1.20 V.3 Atomic force microscopy imaging of the film revealed formation of pyr‑SWCNTs tangles surrounded by globular clusters of (C60‑Pd) and polybisthiothene (Figure 2a). The composite film revealed two potential windows of electrochemical activity, i.e., one at potentials more negative than ~‑0.30 V vs. Ag|AgCl because of the fullerene electroactivity and the other at potentials more positive than ~0.40 V because of the PBT electroactivity (Figure 2b). Cyclic constant-current 90-mA charging and discharging at different voltage limits of the pyr‑SWCNTs|(C60‑Pd)‑PBT film coated Au disk electrode was reversible and pseudo-linear for the voltage range not exceeding 1.60 V (Figure 2c).
-
Pieta, P.; Obraztsov, I.; D'Souza, F.; Kutner, W. Composites of Conducting Polymers and Various Carbon Nanostructures for Electrochemical Supercapacitors. ECS J Solid State Sci 2013, 2, M3120-M3134.
-
Pieta, P.; Grodzka, E.; Winkler, K.; Warczak, M.; Sadkowski, A.; Zukowska, G. Z.; Venukadasula, G. M.; D'Souza, F.; Kutner, W. Conductive, Capacitive, and Viscoelastic Properties of a New Composite of the C-60-Pd Conducting Polymer and Single-Wall Carbon Nanotubes. J Phys Chem B 2009, 113, 6682-6691.
-
Pieta, P.; Venukadasula, G. M.; D'Souza, F.; Kutner, W. Preparation and Selected Properties of an Improved Composite of the Electrophoretically Deposited Single-Wall Carbon Nanotubes, Electrochemically Coated with a C-60-Pd and Polybithiophene Mixed Polymer Film. J Phys Chem C 2009, 113, 14046-14058.



